[ad_1]
Pham et al. (2023) uses data on regulatory decisions and health technology assessments (HTAs) in Australia, Canada, and the UK and compares them to the drugs that are FDA-approved in the US. They find that:
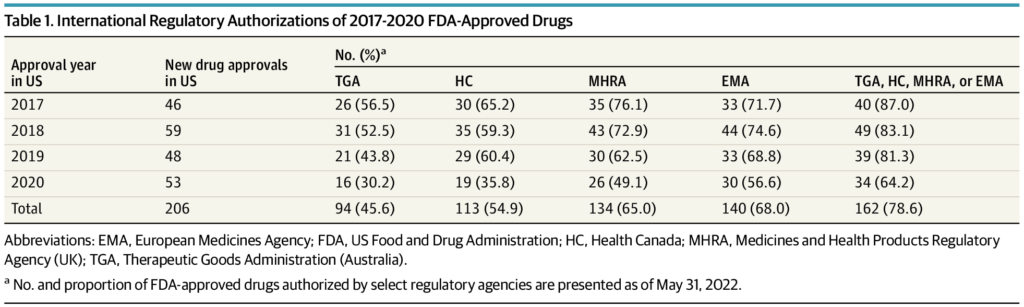
The FDA approved 206 new drugs in 2017 through 2020, of which 162 (78.6%) were granted marketing authorization by at least 1 other regulatory agency at a median (IQR) delay of 12.1 (17.7) months following US approval. Conversely, 5 FDA-approved drugs were refused marketing authorization by an international regulatory agency due to unfavorable benefit-to-risk assessments. An additional 42 FDA-approved drugs received negative reimbursement recommendations from HTA agencies in Australia, Canada, or the UK due to uncertainty of clinical benefits or unacceptably high prices. The median (IQR) US cost of the 47 drugs refused authorization or not recommended for reimbursement by an international agency was $115,281 ($166,690) per patient per year. Twenty drugs were for oncology indications, and 36 were approved by the FDA through expedited regulatory pathways or the Orphan Drug Act.
The full article is here.
[ad_2]
Source link