[ad_1]
One approach CMS has is the Cell and Gene Therapy (CGT) Access Model. Under the CGT Access Model, CMS (at the federal level) will negotiates outcomes based agreements (OBAs) with manufacturers, which will include pricing terms and outcomes to be evaluated. Then states can elect to participate in the contract terms negotiated by CMS or they can opt out and negotiate on their own. From CMS’s perspective, they are hoping that the increased purchasing power will lead to lower prices (or equivalently, more negotiated rebates). For manufacturers, they are hoping that the CGT access model will expedite access for patients and reduce transaction costs since manufacturers would have to negotiate with much fewer than 50 State Medicaid Agencies. For both parties, outcomes-based contracts are burdensome to implement; having CMS will support states in implementing, monitoring, reconciling, and evaluating the financial and clinical outcomes outlined in OBAs
What CGTs are currently planned for inclusion in the CGT Access model and how much will they cost?
Two CGTs for the treatment of sickle cell disease are planned for inclusion in the model. Both were approved in December 2023. “Casgevy” from Vertex Pharmaceuticals and CRISPR Therapeutics had a list price of $2.2 million per patient; “Lyfgenia” from bluebird bio was listed at $3.1 million per patient.
What types of rebates could State Medicaid Agencies receive under the CGT Access Model?
These would include not only the standard Medicaid Drug Rebate Program (MDRP) rebate (a.k.a. Medicaid best price) but also additional guaranteed rebates and volume rebates as well as rebates paid in the future based on patient and population outcomes.
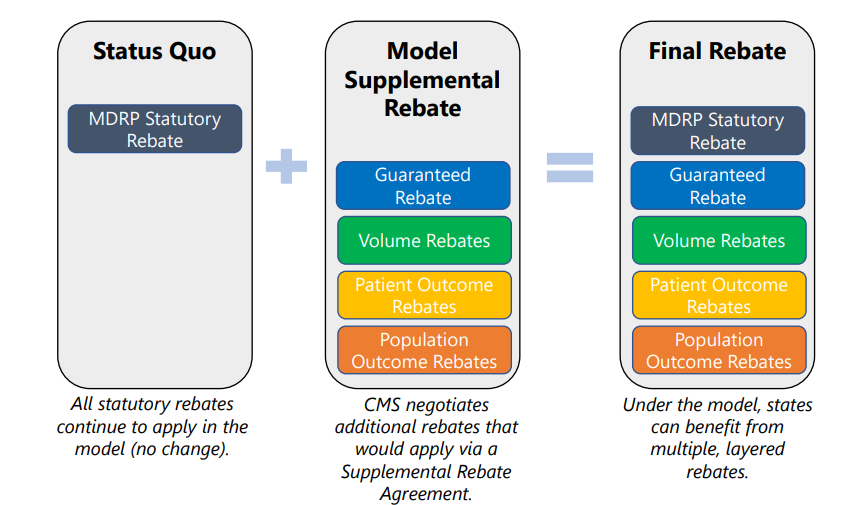
When would the rebates be paid?
The timing of the rebates depends on the type of rebate. Statutory Rebate (MDRP) and the negotiated guaranteed rebate will be paid immediately after treatment. At the end of the year, volume-based rebates could come into effect. Finally, patient-outcome and population-outcome rebates will be paid years after treatment administration. Note that the patient outcome rebates can be paid annually over time to account for cases where patients respond to therapy but perhaps not indefinitely.
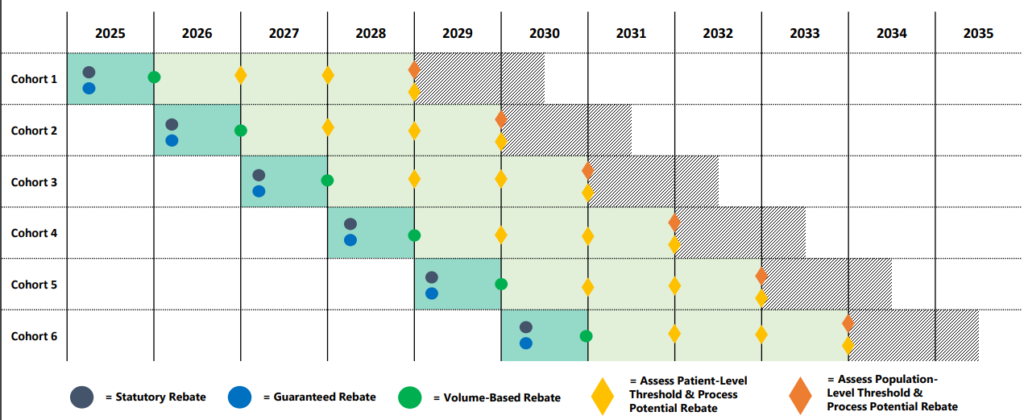
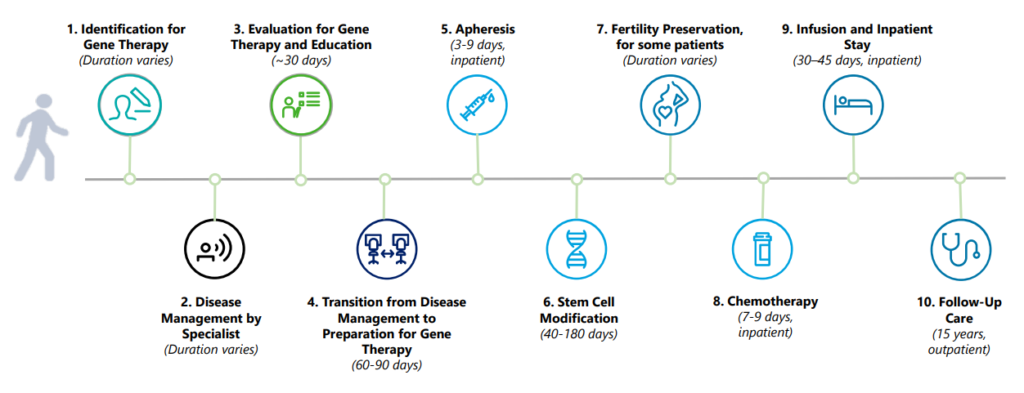
What does the process look like from the patient perspective?
The CGT Access Model: Overview for States presentation has a helpful timeline.
If a state chooses to participate in the model, can it attempt to negotiate additional discounts or terms other than those negotiated by CMS?
If a state opts to participate in the model, it must enter into an agreement with the manufacturer that reflects the terms negotiated by CMS. Some limited state-to-state variation may be permitted, as may be necessary to comport with state legislative and regulatory requirements.
What do states need to do to participate in the CGT Access Model?
Requirements include:
- Execute value-based purchasing supplemental rebate agreements (VBP SRAs) with manufacturers that reflect the Key Terms negotiated by CMS
- Pursue state plan amendments (SPAs) where appropriate
- Establish a standardized access policy for included gene therapies
- Carve included gene therapies out of any inpatient payment bundle
- Require providers to follow requirements for data reporting and claims submissions
- Ensure beneficiaries have access to care with instate or out-of-state qualified gene therapy provider(s)
- Ensure necessary transportation and related travel expenses to beneficiaries (NEMT)
- Meet minimum T-MSIS data requirements
What legislation created the CGT Access Model?
CMS states that:
The CGT Access Model was developed in response to President Biden’s Executive Order 14087, “Lowering Prescription Drug Costs for Americans” and was first proposed in a report responding to the executive order directed by the Secretary of the Department of Health and Human Services.
https://www.cms.gov/priorities/innovation/innovation-models/cgt
How can I learn more?
Some helpful reference material on the CMS CGT Access Model webpage includes:
[ad_2]
Source link