[ad_1]
Back in July, I provided an overview of the Enhancing Oncology Model (EOM). Today I build upon that post focusing largely on CMS’s EOM’s Payment Methodology. I use a Q&A format as well.
What is the goal of EOM?
According to CMS “EOM is a CMMI alternative payment model designed to advance health equity, promote better care coordination, improve access to care, reduce costs, and improve outcomes for Medicare fee-for-service (FFS) beneficiaries with cancer who receive chemotherapy. ”
Which cancer types are eligible for EOM included?
There are seven cancer types eligible for EOM: (i) breast, (ii) chronic leukemia, (iii) lung, (iv) lymphoma, (v) multiple myeloma, (vi) prostate cancer, and (vii) small intestine / colorectal cancer.
Note that low-risk breast cancer (ie, long-term oral endocrine chemotherapy) and low-intensity prostate cancer (indicated by androgen deprivation and/or anti-androgen therapy without chemotherapy) are not included in EOM.
Does EOM only consider chemotherapies?
No! Although all the CMS language uses the term “chemotherapy” frequently, EOM Initiating Therapies include not only traditional chemotherapies (eg, azacitidine, carboplatin, decitabine), but includes immuno-oncology treatments (eg, nivolumab, pembrolizumab) and targeted therapies (eg, bevacizumab, osimertinib, sorafenib? A full list is available on the EOM website here.
Cell and gene therapies–which CMS calls Adoptive Cell Transfer (ACT) therapy–are excluded from the EOM program. For instance, CAR T-cell therapies are excluded from the EOM program.
How long are EOM episodes?
6 months.
Episodes start at a “trigger event” which is the initiation of a Part B or Part D anti-cancer medication. Beneficiaries who continue to receive chemotherapy after completing a six month episode initiate a new episode. Note, however, that “There is no requirement that a chemotherapy-free period exist before the beginning of any episode.” To address the lack of a ‘clean period’ before the trigger event, CMS relies on risk adjustment.
How are episodes assigned to physician practice groups?
This is based on the oncology physician group practice that either (i) provided the first evaluation and management (E&M) service during the 6 month performance period as long as they provided at least 25% of E&M services in the episode, or (ii) the PGP with the plurality of E&M services if the initial PGP did not reach the 25% threshold.
What types of payments can physicians group practices (PGPs) receive?
Practices receive the standard Medicare FFS billing. In addition, EOM has the option to include a Monthly Enhanced Oncology Services (MEOS) payment, which can be thought of as a capitation payment. Base MEOS payments are $70 per month but increase to $100 per month for dual-eligible beneficiaries. Based on treatment cost and quality, PGPs have two-sided risk: high-quality/low cost PGPs can earn a retrospective performance-based payment (PBP), but low-quality/high-cost PGPs can owe a retrospective performance-based recoupment (PBR).
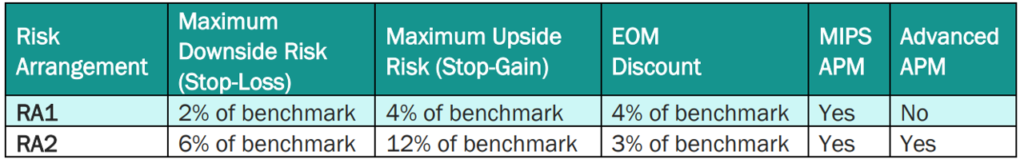
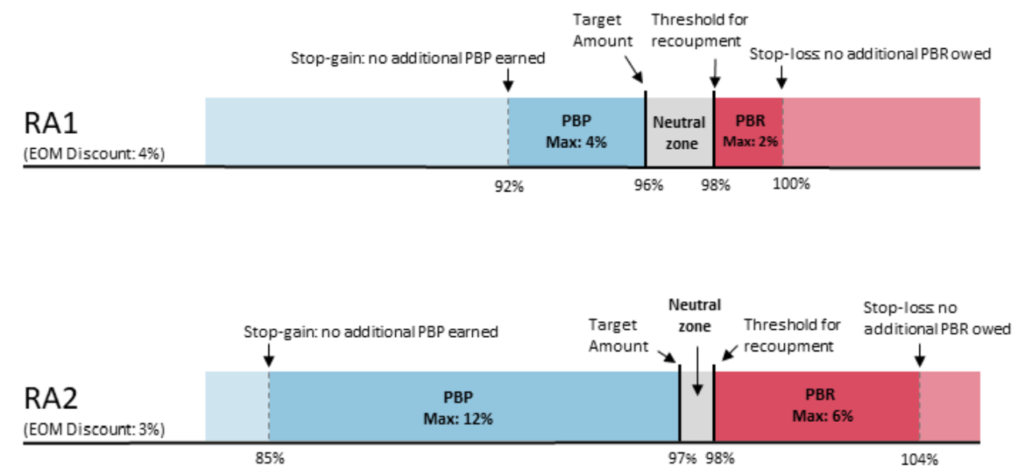
PBP and PBRs depend on the risk model selected. By default, EOM participants and pools are in RA1 unless they request to be in RA2. The payment specifications for RA1 and RA2 are below. RA1 requires PGPs to meet a larger target discount (4%) but has a 2% maximum downside risk; RA2 requires a smaller discount (3%) but PGPs are liable for more positive and negative risk (maximum 6% downside risk). By default, EOM participants and pools are in RA1 unless they request to be in RA2.
What type of expenses are included in an episode?
All Medicare Part A and Part B FFS expenditures (payments) and certain Part D expenditures are counted as part of the episode cost. Specifically, EOM episodes only include the Low-Income Cost Sharing Subsidy (LICS) amount and 80 percent of the Gross Drug Cost above the Catastrophic (GDCA) threshold. All other Part D expenditures are not included in an EOM episode because they are paid on a capitated basis by Part D plans.
How does EOM account for outlier episodes and differences in patient characteristics?
To reduce the likelihood that a few very high or very low cost episodes will lead to a poor performance score, CMS winsorizes episode expenditures at the 5th and 95th percentiles.
Additionally, CMS risk adjusts episode costs based on factors such as demographics (ie, age, sex), income (ie, dual eligibility, Part D LIS eligibility), comorbidities (autoimmune disorders, COPD, dementia, endocrine disorders, hearing disease, hematologic disease , hypertension), count of other HCCs, receipt of specific services (ie, cancer-directed surgeries, bone marrow transplant, radiation), institutional status, participation in a clinical trial, prior chemotherapy use, and episode length (eg, if died or unrolled)
obesity. Risk adjustment is based 50% on national cancer-specific cost predictions and a mix of regional and PGP specific cost estimates with larger PGPs having more weight on their historical data and smaller PGPs having more weight on regional cost predictions.
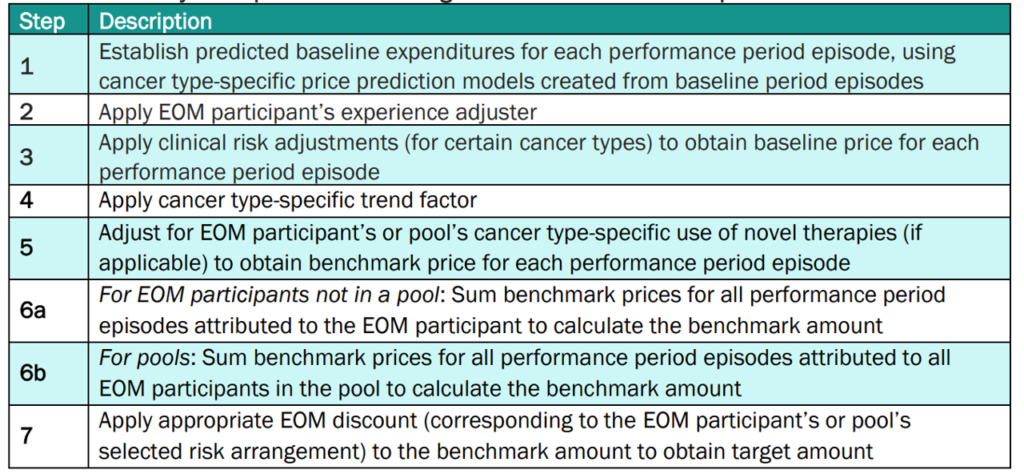
More details on how CMS calculates the target amounts for EOM participant PGPs is below.
Does EOM account for tumor stage or tumor mutations?
Yes, but crudely. There is a clinical adjuster if the patient ever had metastatic disease (currently only used for breast, lung and small intestine/colorectal cancers), and a single biomarker is adjusted for (HER2+ patients with breast cancer).
How does EOM deal with new treatments?
EOM could potentially de-incentivize the use of clinically beneficial but high cost treatments. To not unduly disincentivize innovation, EOM includes a new therapy adjustment where oncology drugs are considered “new” for 2 years from FDA approval for that specific indication.
Does EOM account for quality of care?
Somewhat. EOM does this through Aggregate Quality Score (AQS). In short, PGPs are only eligible to receive bonuses if they meet a minimum threshold on the AQS.
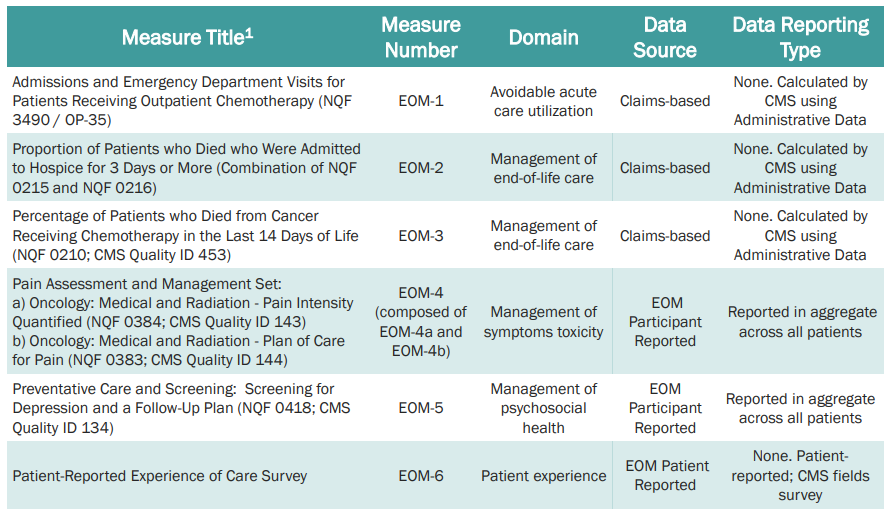
Quality of care includes certain activities (24/7 access to care, patient navigation, documented care plan, following clinical guidelines, conducting health-related social needs screening, and use of certified electronic health records technology (CEHRT). The specific quality measures are listed below based on CMS’s Quality Strategy Webinar from August 25, 2022.
For more details on EOM as it evolves, see the CMS’s EOM website.
[ad_2]
Source link



