[ad_1]
The FDA wants drugs to be more targeted to factors that impact patient’s lives in the ways that they care about. To achieve this goal, last month the FDA released a third guide document on patient focused drug development generally applicable to a variety of clinical outcome assessments (COAs), including patient-reported outcome (PRO), observer-reported outcome (ObsRO), clinician- reported outcome (ClinRO), and performance-based outcome (PerfO) measures.
- Patient reported outcomes (PRO). Reports come directly from the patient. These measures are useful for assessment of symptoms (eg, pain intensity, shortness of breath), functioning, events, or other aspects of health from the patient’s perspective. Often PROs are collected through questionnaires, but increasingly are being collected using digital health technologies (DHTs).
- Observer-reported outcomes (ObsRO). Reports come from someone other than the patient or a health professional (eg, a parent or caregiver) who has the opportunity to observe the patient in everyday life. These measures are used when patients such as young children cannot reliably report for themselves, or to assess observable aspects related to patients’ health (eg, signs, events, or behaviors).
- Clinician-reported outcomes (ClinRO). Reports come from a trained health-care professional using clinical judgment. This type of measure is useful when reports of observable signs, behaviors, clinical events, or other manifestations related to a disease or condition benefit from clinical judgment or expertise.
- Performance outcome (PerfO). These measures are based on standardized task (s) actively undertaken by a patient. Examples includ grip strength test or six minute walk distance (6MWD).
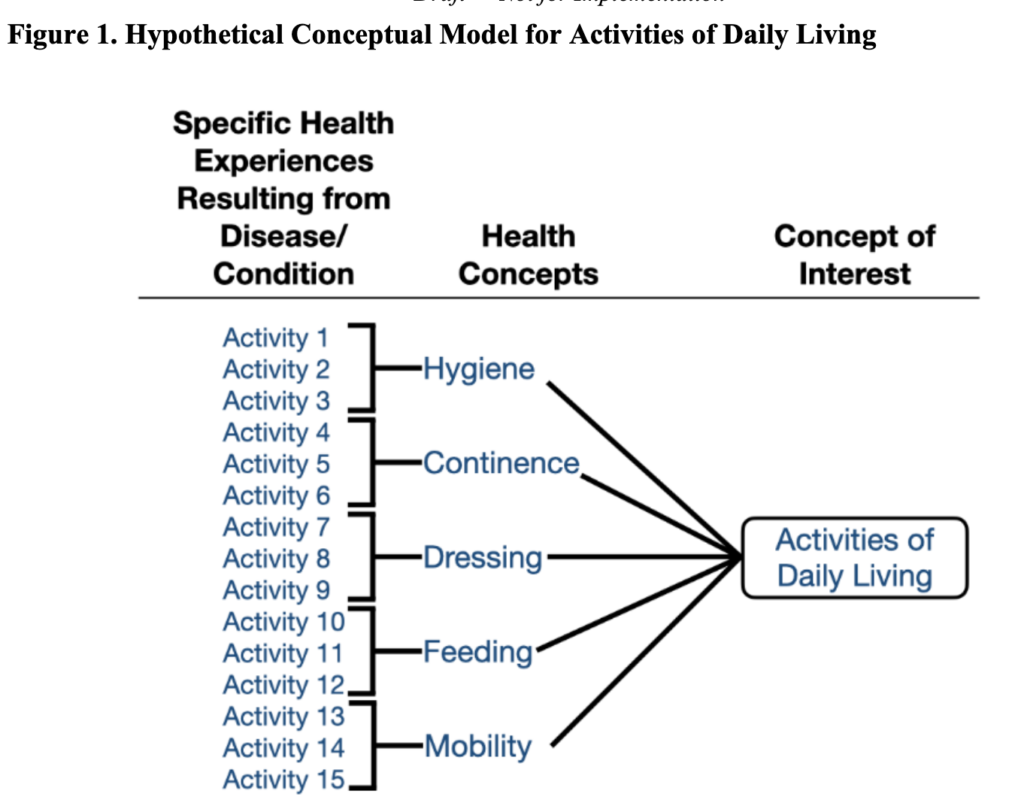
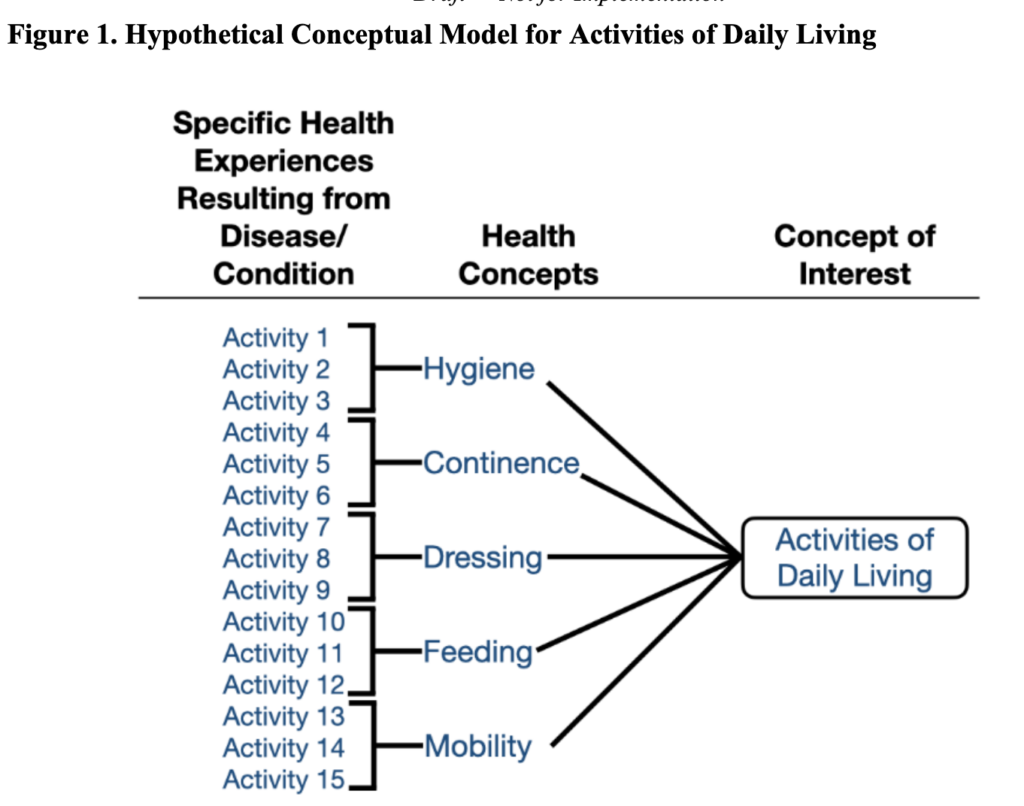
FDA recommends that a conceptual model be created to represent how patients’ specific health experiences from their disease / condition relate to measures of interest. An example is below.
The context of how measures are used is also important. Specifically:
- Use of the COA: Clinical trial objectives and how the COA will be used to support COA-based endpoints (eg, computing the mean COA score at 12 weeks)
- Target Population: Including a definition of the disease or condition; participant selection criteria for clinical trials (eg, baseline symptom severity, patient demographics, comorbidities); and expected patient experiences or events during the trial (eg, that some patients will require assistive devices)
- Study Context: The clinical trial design in which the COA is to be used, including the type of comparator group and whether those providing responses or participating in the tasks for the COA (patients, observers, clinicians, trained raters) are masked with respect to treatment assignment and / or study visit)
- Timing of when assessment (s) of the COA is conducted269
- COA Implementation: Including the site for COA collection (eg, inpatient hospital, outpatient clinic, home); how the COA will be collected (eg, DHT, paper form); and by whom (eg, patient, study coordinator, investigator, parent / caregiver.)
The FDA also provides a roadmap for selecting fit-for-purpose COA for use in a clinical trial.
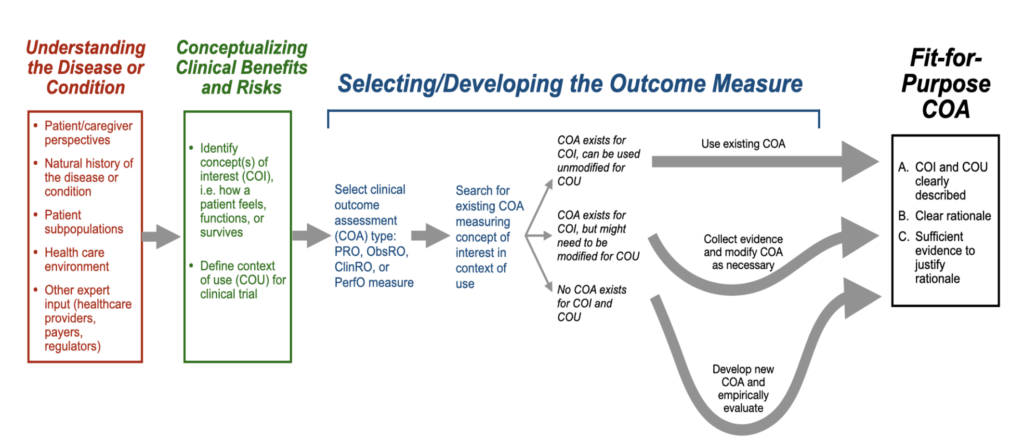
Once the roadmap is followed, manufacturers need to justify the inclusion of a COA in a clinical trial based on 8 key points of evidence.

For more information on the roadmap and the specific evidence needed to rationalize the inclusion of a COA in a clinical trial, do real the full FDA guidance here.
[ad_2]
Source link